Une avancée majeure en Chine : la première guérison du diabète de type 1 au monde
Une avancée majeure en Chine : la première guérison du diabète de type 1 au monde
Une avancée médicale révolutionnaire
Pour la première fois, une jeune femme chinoise de 25 ans a entièrement guéri du diabète de type 1 grâce à une thérapie développée par des chercheurs chinois. Cette approche, menée à l’hôpital principal de Tianjin en collaboration avec l’Université de Pékin, est fondée sur la régénération cellulaire. Les chercheurs ont prélevé des cellules du pancréas de la patiente, qu’ils ont ensuite traitées chimiquement de manière à les faire retrouver un état de cellules souches. Une fois modifiées, les cellules ont été ré-implantées, par voie micro-invasive, dans le pancréas de la patiente. A la suite de cette opération, le taux d’insuline sécrétée a augmenté de 700 %. Le résultat de cette opération furent rapides et durables : dès 75 jours après l’intervention, le taux de glycémie se régulait sans besoin d’injection d’insuline extérieure, et la patiente ne présentait plus aucun des symptômes du diabète. Cette guérison complète marque une révolution thérapeutique majeure.[1]
Une épidémie silencieuse
Malgré cette avancée, le diabète demeure une véritable urgence de santé publique. Avec plus de 140 millions de personnes touchées, la Chine enregistre l’une des prévalences les plus élevées au monde, représentant environ 12 % de sa population adulte[2]. D’ici 2040, la Chine pourrait compter 166 millions de diabétiques, consolidant sa place parmi les pays les plus touchés.[3] Fait préoccupant, le diabète de type 2 touche également 1,77 % des enfants chinois âgés de 3 à 18 ans[4]. À cela s’ajoute une prise de conscience insuffisante : seulement 36,5 % des diabétiques chinois connaissent leur état et, parmi ceux traités, moins de la moitié (49,2 %) réussissent à contrôler leur glycémie efficacement[5]. Or les complications liées au diabète sont nombreuses et peuvent être graves[6] :
- Maladies cardiovasculaires : principal facteur de mortalité chez les diabétiques.
- Problèmes rénaux : l’insuffisance rénale est fréquente.
- Atteintes neurologiques et oculaires : des neuropathies, pouvant conduire à la cécité, sont également courantes.
Les causes sous-jacentes de cette épidémie
Le diabète en Chine n’est pas uniquement une question de prédisposition génétique. Plusieurs facteurs sociaux, économiques et environnementaux convergent pour alimenter cette crise.
Urbanisation
Le développement rapide des villes en Chine a transformé les modes de vie. Les populations urbaines, qui représentent une part croissante de la société chinoise, affichent une prévalence de diabète bien supérieure à celle des zones rurales (12,1 % contre 8,3 %).
Changements alimentaires
La modification des habitudes alimentaires a également joué un rôle crucial. La consommation accrue de sucres, d’aliments transformés et de fast-foods au sein des populations aisées expose davantage au risque de diabète. Cette transition, autrefois limitée aux grandes villes, s’étend maintenant aux zones rurales en raison de l’accès croissant aux produits alimentaires industriels.
Sédentarité et stress
Avec l’essor des emplois de bureau, l’activité physique a considérablement diminué. Parallèlement, l’environnement urbain et les défis socioculturels croissants génèrent un stress chronique, connu pour perturber les mécanismes hormonaux et métaboliques.
Vieillissement de la population
A l’instar de nombreux pays, la population chinoise vieillit rapidement. Ce phénomène amplifie mécaniquement la prévalence du diabète, une maladie qui touche davantage les personnes âgées.
Solutions stratégiques : prévenir plutôt que guérir
Pour parvenir à enrayer l’épidémie de diabète, la Chine investit dans des politiques de prévention et de sensibilisation :
Éducation et dépistage
En augmentant la sensibilisation au diabète, il est possible d’augmenter le taux de dépistage précoce et la prise en charge adaptée.
Encourager une alimentation équilibrée
Promouvoir des régimes alimentaires riches en fibres et faibles en sucres contribue à améliorer la santé des populations à risque.
Promotion de l’activité physique
Intégrer l’exercice dans la routine quotidienne via des incitations, la promotion des bienfaits du sport, la création d’infrastructures plus accessibles dans les zones urbaines pourrait contribuer à réduire la prévalence de la maladie.
Renforcement des infrastructures médicales et de leur équipement
Les régions rurales manquent encore de moyens pour diagnostiquer et traiter efficacement le diabète. Renforcer les capacités des centres de soins locaux est essentiel pour combler cet écart. Des investissements massifs dans les appareils de gestion du diabète tels que glucomètres, pompes à insuline et autres dispositifs médicaux innovants sont réalisés pour répondre à ce défi de santé publique.[7]
Un terrain d’opportunités pour les entreprises étrangères
La lutte contre le diabète en Chine offre de nombreuses opportunités pour les entreprises européennes et françaises, notamment dans :
- Les dispositifs médicaux innovants comme les glucomètres connectés.
- Les solutions numériques pour le suivi et la gestion du diabète.
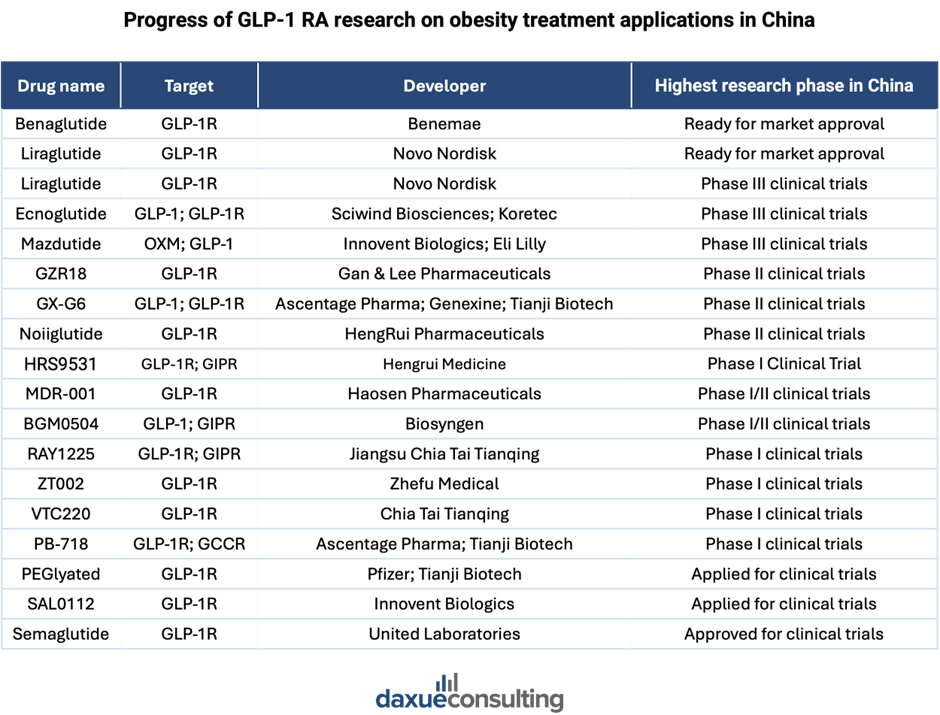
- Les traitements avancés (tels que les médicaments GLP-1 comme l’Ozempic), tant contre le diabète lui-même que contre ses complications.
La clé du succès réside dans une compréhension approfondie du marché local, des acteurs et des réglementations en vigueur. VVR Medical, département de VVR International, expert des marchés pharmaceutiques et médicaux chinois, accompagne les entreprises dans leur stratégie de développement en Chine. N’hésitez pas à nous contacter à l’adresse contact@vvrmedical.com pour recevoir les conseils et le soutien de nos experts dans votre projet.
[1] Première mondiale : une Chinoise guérit du diabète de type 1
[2] https://www.federationdesdiabetiques.org/information/diabete/chiffres-monde
[3] https://fr.statista.com/statistiques/571668/pays-avec-le-plus-grand-nombre-previsionnel-de-personnes-diabetiques-en-2040/
[4] https://www.thelancet.com/journals/lanpub/article/PIIS2468-2667(24)00250-0/fulltext
[5] https://www.dbl-diabete.fr/tout-sur-le-diabete/societe/classement-diabete-pays-monde
[6] https://www.thelancet.com/journals/lanpub/article/PIIS2468-2667(24)00250-0/fulltext
[7] https://www.mordorintelligence.com/fr/industry-reports/china-diabetes-devices-market/market-size